 This website is best viewed
This website is best viewed
using the horizontal display on
your tablet device.
Healthcare Professionals only.
ENGLISH
FRANÇAIS
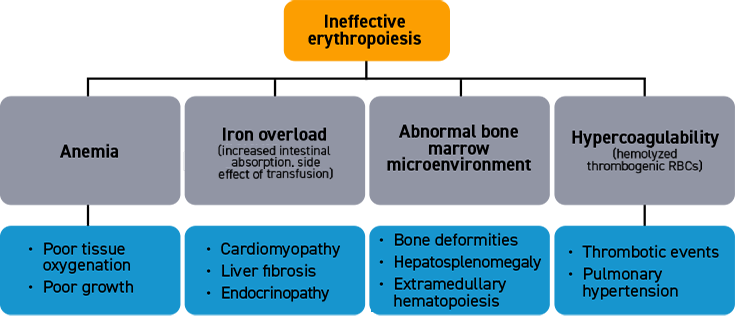
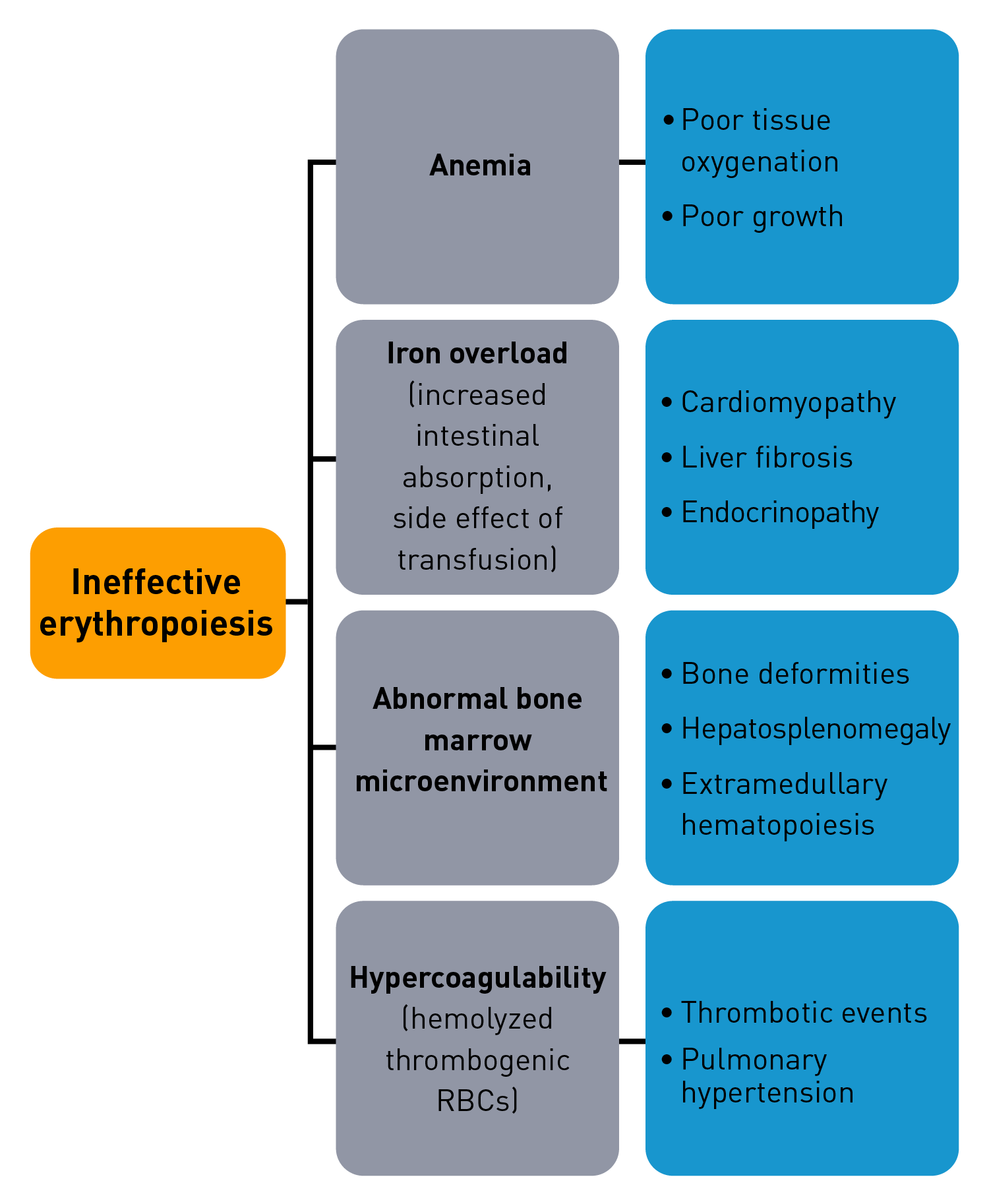
Characteristics of ineffective erythropoiesis
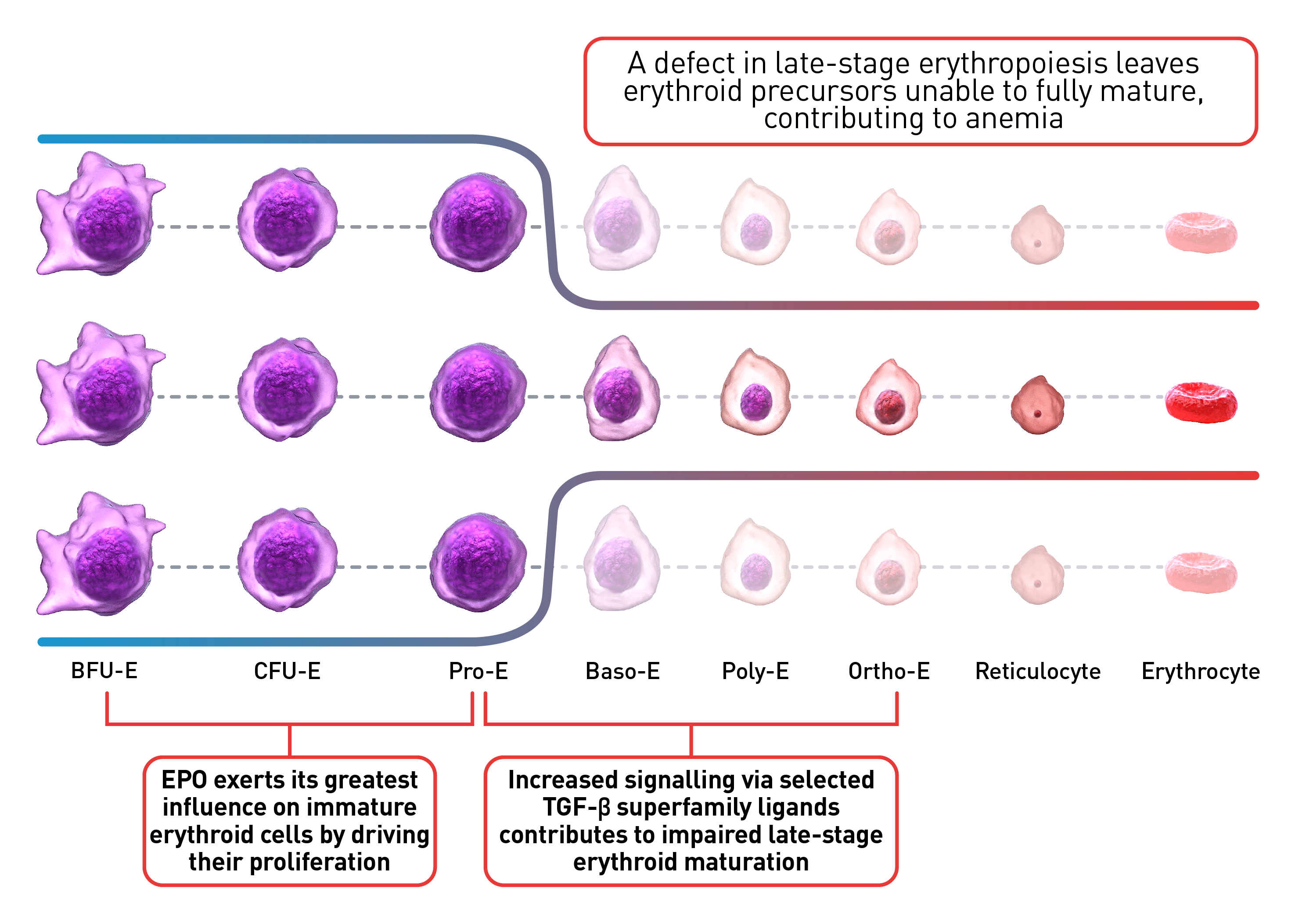
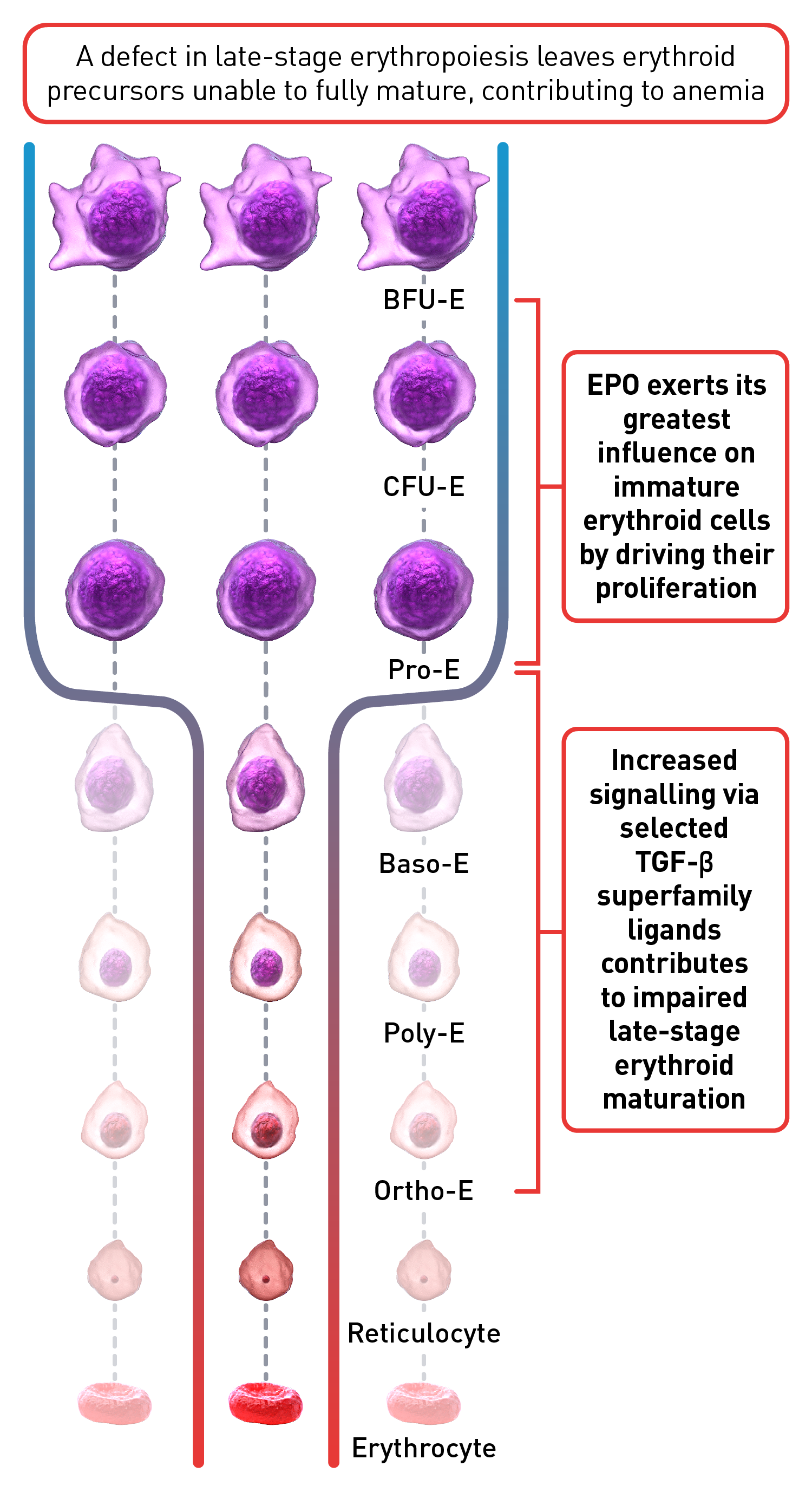
Normal erythropoiesis proceeds with tight regulation of maturation and apoptosis to maintain red blood cell homeostasis and normal oxygen levels.4 However, ineffective erythropoiesis is an ongoing pathological state where increased erythroid proliferation is unable to restore red blood cell count due to increased apoptosis of immature erythroid cells and impaired late-stage maturation.1,4
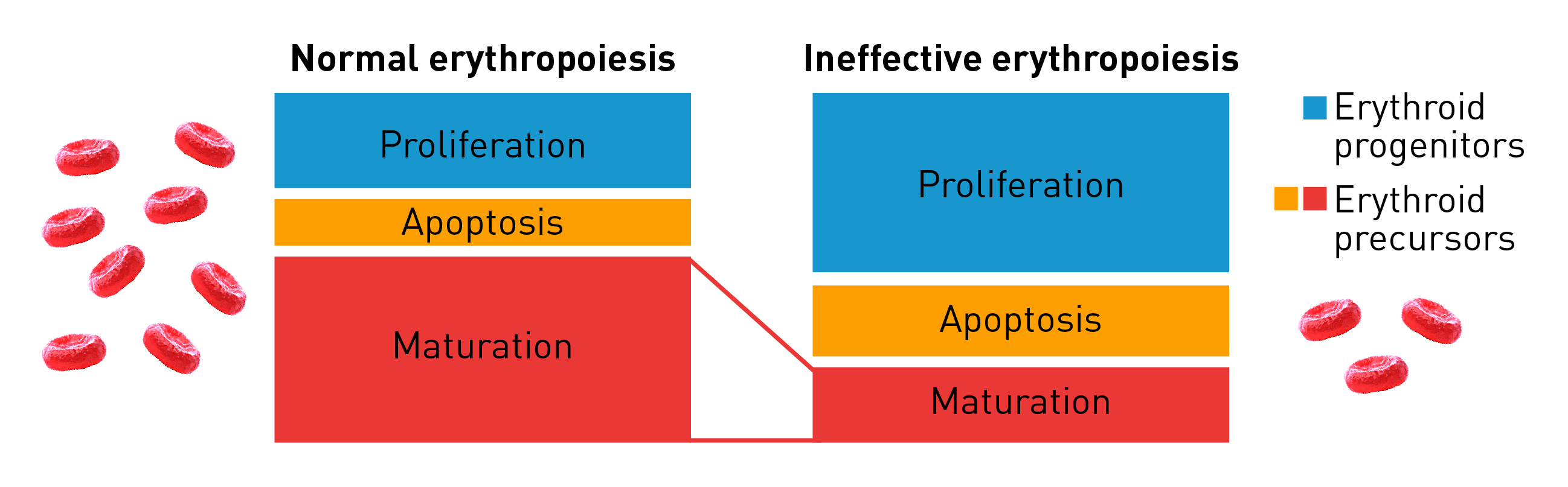
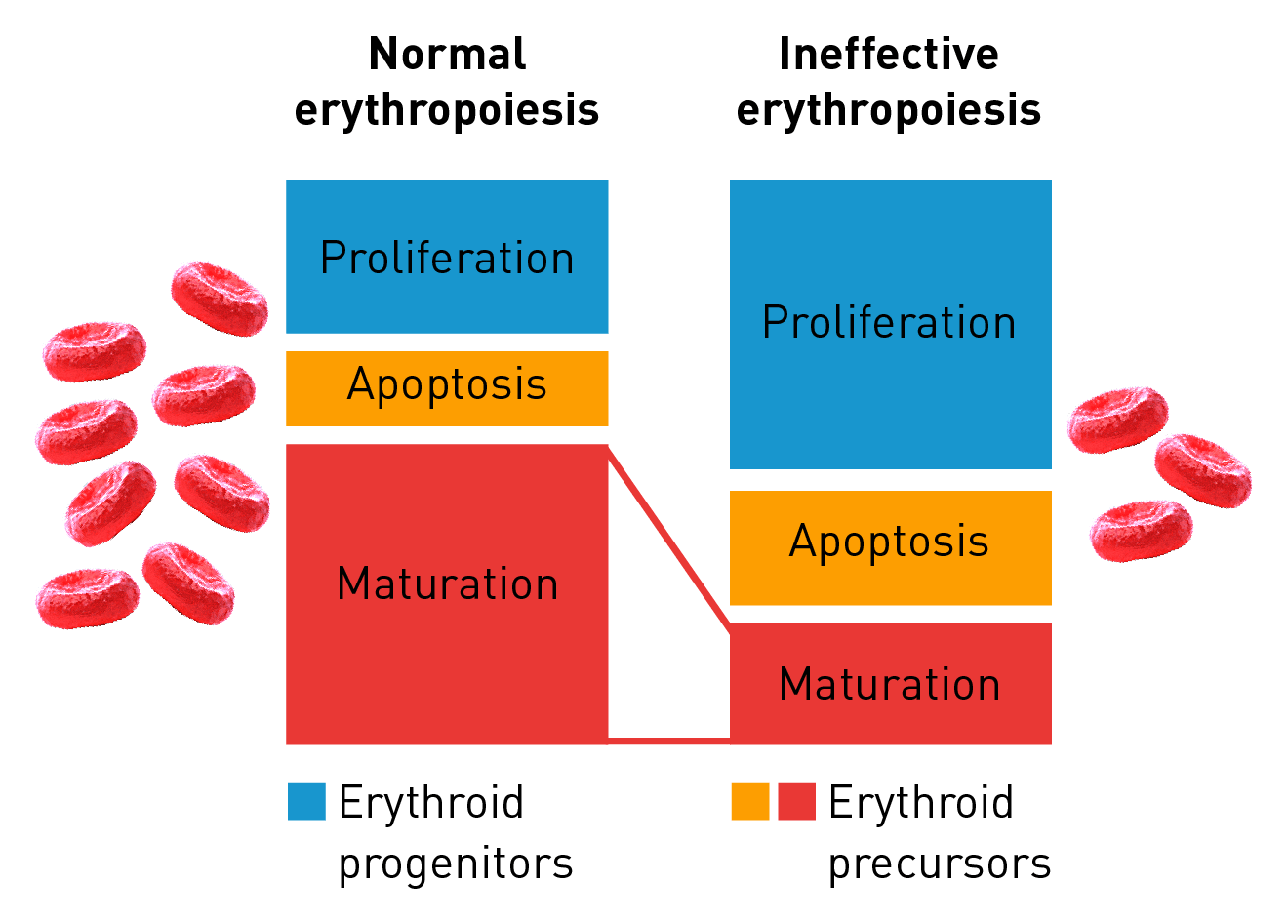
RBC: Red blood cells, HSP: Heat shock protein, Baso‑E: Basophilic erythroid, BFU‑E: Burst-forming unit-erythroid, CFU-E: Colony-forming unit-erythroid, EMD: Erythroid maturation defect, EPO: Erythropoietin, Ortho-E: Orthochromatophilic, Poly‑E: Polychromatophilic, Pro‑E: Proerythroblasts, TGF: Transforming growth factor.
 This website is best viewed
This website is best viewed
using the vertical display on
your mobile device.